The “Ins and Outs” of ER–Chloroplast Lipid Trafficking
Karki et al. revisit the long-standing question of how and where lipid trafficking occurs during galactolipid synthesis. They report evidence for metabolically distinct pools of phosphatidylcholine, suggesting an underlying spatial organization in the ER–chloroplast metabolic interactions. Plant Cell https://doi.org/10.1105/tpc.19.00121
By Philip Bates
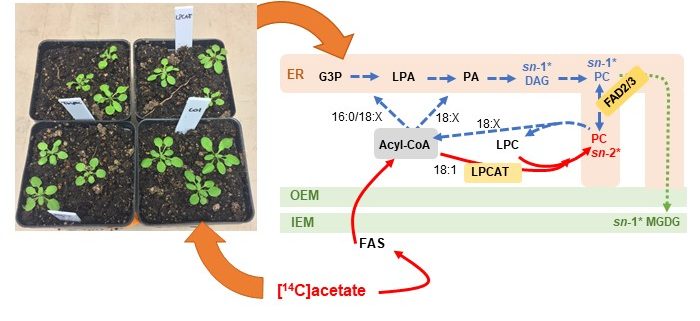
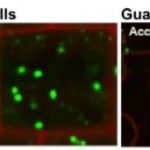
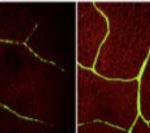
Background: Chloroplast membranes composed predominantly from galactolipids are critical for the organization and compartmentalization of essential photosynthetic reactions in leaves. To synthesize galactolipids, fatty acids are synthesized in the chloroplast stroma, exported out of the chloroplast and assembled into membrane lipid phosphatidylcholine (PC) in the endoplasmic reticulum (ER), and then PC is partially disassembled to produce a substrate that is imported back into the chloroplast for final conversion to galactolipids. This complicated metabolic pathway of galactolipid production has been investigated for over 40 years; however, the lipid substrates transported between the ER and the chloroplast are unclear. Current evidence has suggested the ER-to-chloroplast trafficking of either phosphatidic acid, diacylglycerol, PC, or lysophosphatidylcholine (LPC) for galactolipid synthesis.
Question: We wanted to test the hypothesis of LPC transport from the ER to the chloroplast for galactolipid biosynthesis, which requires chloroplast-localized lysophosphatidylcholine acyltransferase (LPCAT) enzymes. Therefore, we followed the transfer of newly synthesized radiolabeled fatty acids into and out of different lipids over time within Arabidopsis thaliana LPCAT mutants and control leaves.
Findings: The LPCAT mutations eliminated most of the chloroplast-associated LPCAT activity in leaves and redirected the pathway of newly synthesized fatty acid assembly into ER lipids. However, the transfer of the galactolipid substrate derived from PC to chloroplasts was not affected. This suggests that chloroplast associated LPCAT activity is predominantly involved in incorporation of newly synthesized fatty acids exported from the chloroplast into PC. In addition, we characterized metabolically distinct pools of PC involved in chloroplast fatty acid export and PC turnover for galactolipid production, suggesting spatial separation of PC metabolism for lipid trafficking between the chloroplast and ER.
Next Steps: We have narrowed down the ER-to-chloroplast lipid trafficking candidates for essential galactolipid synthesis, and we demonstrated that the mutant lines characterized here simplify metabolic tracing of the PC–galactolipid precursor–product relationship. Combining these mutants, with mutants or overexpressor lines in phosphatidic acid or diacylglycerol metabolism, and further analysis through metabolic tracing may finally identify the elusive lipid substrates transferred from the ER to the chloroplast for galactolipid production.
Nischal Karki, Brandon S. Johnson, and Philip D. Bates. (2019). Metabolically Distinct Pools of Phosphatidylcholine Are Involved in Trafficking of Fatty Acids out of and into the Chloroplast for Membrane Production. Plant Cell. https://doi.org/10.1105/tpc.19.00121.
Key words: lipid, metabolism, biochemistry, chloroplast, endoplasmic reticulum, trafficking