Sterol-binding activity of PR-1 contributes to its antimicrobial activity ($)
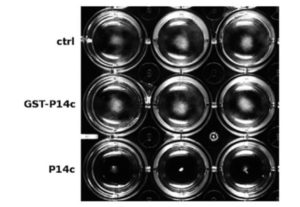 PATHOGENESIS-RELATED 1 (PR-1) protein was identified 50 years ago as a small protein induced in response to pathogens, but its mode of action has remained obscure. PR-1 is a member of the CAP family (cysteine-rich secretory protein, antigen 5, and pathogenesis-related 1). These proteins share a 150 amino acid domain that forms an α-β-α sandwich fold. Recently, yeast CAP proteins were found to bind sterols. Gamir et al. showed that PR-1 also can bind sterols, and that expression of PR-1 can complement yeast CAP mutants. Phytophthora are unable to synthesize sterols and rely on their uptake from the medium; these oomycetes are also much more sensitive to PR-1 than fungal pathogens. The authors showed that PR-1 can inhibit growth of Phytophthora brassicae in vitro, but that this inhibition is eliminated in the presence of excess cholesterol in the growth medium. PR-1 mutants blocked in sterol binding, or fusion proteins rendered too large to pass into the pathogen cell lack antimicrobial activity. Together, these studies suggest that PR-1’s mode of action is through direct removal of sterols from pathogens. Plant J. 10.1111/tpj.13398
PATHOGENESIS-RELATED 1 (PR-1) protein was identified 50 years ago as a small protein induced in response to pathogens, but its mode of action has remained obscure. PR-1 is a member of the CAP family (cysteine-rich secretory protein, antigen 5, and pathogenesis-related 1). These proteins share a 150 amino acid domain that forms an α-β-α sandwich fold. Recently, yeast CAP proteins were found to bind sterols. Gamir et al. showed that PR-1 also can bind sterols, and that expression of PR-1 can complement yeast CAP mutants. Phytophthora are unable to synthesize sterols and rely on their uptake from the medium; these oomycetes are also much more sensitive to PR-1 than fungal pathogens. The authors showed that PR-1 can inhibit growth of Phytophthora brassicae in vitro, but that this inhibition is eliminated in the presence of excess cholesterol in the growth medium. PR-1 mutants blocked in sterol binding, or fusion proteins rendered too large to pass into the pathogen cell lack antimicrobial activity. Together, these studies suggest that PR-1’s mode of action is through direct removal of sterols from pathogens. Plant J. 10.1111/tpj.13398







Leave a Reply
Want to join the discussion?Feel free to contribute!