Seeing the Cell Wall in a New Light
Sidney L. Shaw
Dept. of Biology (and Physics), Indiana University, Bloomington, IN 47405
sishaw@indiana.edu
How cell expansion is controlled to achieve a specific cell morphology remains one of the frontier questions in plant biology. The carefully guided extension of the plant cell wall produces a remarkable variety of cell shapes, and in a larger context, creates the physical frame for the plant itself. Flowering plants generate new cells through divisions from meristematic stem cells with subsequent divisions following geometric and developmental rules (Steeves and Sussex, 1989). Each cell undergoes a lineage-specific expansion, controlled by developmental factors but strongly influenced by environmental cues. Deceptively simple shapes, like the rectangular cells found in roots and shoots, grow primarily through elongation of connected sidewalls, leaving the apical and basal cell faces almost static (Gendreau et al., 1997). More complex cell forms, like trichomes, guard cells, and the puzzle-piece-like cells found in the leaf epidermis, show combinations of diffuse expansion and polar growth (Szymanski and Cosgrove, 2009). Understanding the mechanisms directly affecting cell shape requires a multidisciplinary approach, integrating genetic and cell biological data with biomechanical studies of the cell wall. In this Issue of Plant Physiology, Altartouri et al. (2019) introduce Brillouin microscopy to the experimental tool box for studying cell shape formation.
Cell wall mechanics and the mechanisms driving plant cell growth have a long and rich experimental history (Baskin, 2005; Cosgrove, 2014). The dominant narrative emerging from decades of biochemical and biophysical studies places cellulose microfibrils in the principal role governing the material properties of the wall, with hemi-celluloses providing crosslinks and pectic polysaccharides acting mostly as filler (Carpita and Gibeaut, 1993; Mccann and Roberts, 1994). The length scale and observed co-alignment of the extruded cellulose microfibrils provide a natural explanation for many of the wall’s mechanical properties. Crystalline cellulose is structurally rigid and can be crosslinked into a mesh that takes on important physical properties related to the number of crosslinks, the length distribution of the fibrils, and the degree of fibril co-orientation (Eichhorn and Young, 2001). Experiments showing that perturbations to cellulose deposition produce more isotropic cell shapes led to the idea that co-oriented cellulose fibrils impart a material anisotropy to the cell wall, resulting in anisotropic cell expansion and the formation of non-spherical cell shapes (Baskin, 2005). Further discoveries showing that microtubules at the cell cortex can influence how cellulose is patterned into the wall (Paredez et al., 2006), and that secreted proteins (e.g., XETs, expansins) alter cellulose crosslinking in response to wall acidification (Cosgrove, 1998), extended this narrative to show how plant cells could potentially orient their cellulose microfibrils and activate growth to achieve specific shapes (Cosgrove, 1998; Van Sandt et al., 2007; Cosgrove, 2016).
Genetic screens, initially investigating how cell wall polymers are made, led to the fascinating discovery that pectins play substantial structural roles in the plant cell wall and do not simply control wall porosity (Micheli, 2001). Pectins include a narrow class of relatively short chain polysaccharides that are secreted into the wall in a soluble, methyl-esterified form. De-esterification leads to precipitation and calcium-dependent gelation in the cell wall. Pectin gels are relatively weak, compared to cellulose, and the length scale of individual molecules does not provide an obvious mechanism for creating materials with anisotropic properties. Hence, structural roles for pectins have long been doubted as significant for plant cells. Indeed, softer secreted materials (e.g., callose) are used structurally in lieu of pectins for constructing new cell plates, for extending pollen tubes, and for defense-related emergencies (Chen and Kim, 2009). Genetic manipulations of enzymes that control the degree of pectin esterification clearly show, however, that cell shape, and even anisotropic cell shapes, depend on the correct placement and chemical modification of pectic polysaccharides (Peaucelle et al., 2012; Peaucelle et al., 2015).
The idea that pectins are a structural element in the plant cell wall, hypothesized to function in the genesis of cell shape, upsets a long-held narrative and provides much needed grist for a tired mill. One specific case that is certain to provide a challenge for both the new pectinites and the more traditional cellulosers to explain is how lobing occurs in leaf epidermal cells. Pavement cells start out with relatively indistinct shapes, but form interlocking lobes with neighboring cells as the leaf expands, increasing the adjoining surface area. The formation of these puzzle-piece shapes has been posited to occur through a variety of mechanisms (Panteris and Galatis, 2005; Szymanski, 2014; Sapala et al., 2018; Vofely et al., 2019) guided by either local differences made in the strength of the anticlinal wall materials (i.e., the walls attached to each other) or the relief of stresses in the outer-facing periclinal cell walls (Fu et al., 2005; Panteris and Galatis, 2005; Armour et al., 2015; Higaki et al., 2017; Majda et al., 2017; Elsner et al., 2018). The composition and organization of both the cellulose and pectic polysaccharides change over the course of lobe initiation and maturation. How these changes relate to the mechanical properties of the cell wall remains a challenging question and highlights specific gaps in our global understanding of cellular morphogenesis. For example, the two adjoining pavement cells are bound together by pectins and certain hemicellulose molecules where the cell on one side cannot ‘grow’ its cell wall without reciprocal expansion of the wall from the neighboring cell. This is a long-held tenet of the plant biology world stemming from the requirement that the cell walls form a structurally stable framework for the plant and do not slip relative to each other. Moreover, plant cells are not known to endure ingrowths from other cells that would lead to an indention and reduction in cell volume. So how do two adjacent pavement cells expand their shared walls such that both develop lobes that appear to have grown into the neighboring cell?
Characterizing the mechanical properties of the cell wall at a cellular level is one of the larger challenges facing plant cell biologists. Cytological probes have been developed for assessing the chemical composition of fixed and, to a lesser degree, living cells (Anderson et al., 2010; Gonneau et al., 2012). However, simply understanding wall composition or relative thickness are only weak proxies for inferring mechanical properties. Traditionally, cell wall mechanics have been couched in classical stress/strain relations, with an elastic (reversible) component and a more plastic (irreversible) extension realized after a threshold strain is reached in response to the stress of turgor pressure (Cosgrove, 2016). When expressed in more than one dimension, the mechanical formulae for wall extension become quite complicated and the distribution of stresses becomes locally dependent on cell shape (Dumais et al., 2006). Even the most ambitious models developed to explore how the material properties of the wall relate to cell expansion are forced, for lack of information, to reduce these complexities to steady state factors or parameters representing the macroscopic compliance of the wall. For a growing cell, the wall materials are straining against the stress of turgor, where the local rates of extension depend on some set of bonded carbohydrate chains, dynamically altered by the act of extension, by additions of new materials, and through changes in cell wall chemistry. Not an intractable problem, but not a place for those who thought the cell wall expansion was boring (i.e., mostly wet cardboard (Sussman, 2019)).
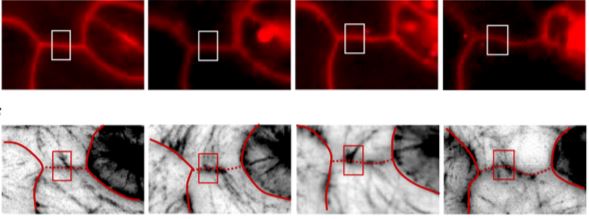
New non-invasive methods for assessing the physical properties of walls in living cells are desperately needed. Spectroscopic methods have been applied to plant cell walls for decades, mostly in the form of Fourier Transform Infra-Red (FTIR) imaging (McCann et al., 1992) and polarized light microscopy (Green, 1962). More recently, non-linear and pump-probe methods have entered the arena (Cox et al., 2005; Mansfield et al., 2013) and, in the manuscript from Altartouri et al., Brillouin microscopy (Scarcelli et al., 2015). This later development holds particular promise for plant biologists wanting to measure the material stiffness of the wall without physically or chemically perturbing the growing cell. Brillouin spectroscopy is related to the more familiar Raman spectroscopy. With Raman, a range of infra-red wavelengths are used to probe the material where the majority of the light passes through or scatters without changing wavelength/frequency. However, if the energy at a particular probe wavelength matches the energy of the molecular vibrations within a material, a small amount of the energy is scattered in a different capacity, yielding photons of slightly lower or higher wavelength. A Raman spectrum, measuring light of shifted wavelength/frequency at each IR probe step, can therefore be diagnostic for the molecular composition of a particular material and its energy state. Brillouin spectroscopy uses light of lower energy to probe vibrations that are set up in materials interacting with the light, referred to as ‘phonons’ in material science. While Brillouin spectroscopy takes advantage of the same ‘inelastic’ scattering process as Raman, leading to photons of slightly higher or lower energy than the probe beam, the meaning is substantively different. Brillouin scattering changes with the elasticity of the material and tends to shift when the material is compressed or stretched owing to the change in bulk elastic modulus (Scarcelli et al., 2015). That is a very useful tool if applied to biological materials like the lens of the eye or the wall of an expanding plant cell (Scarcelli et al., 2011; Scarcelli et al., 2015; Elsayad et al., 2016). When adapted to a laser scanning microscope platform as an imaging apparatus (Scarcelli and Yun, 2007), Brillouin microscopy provides non-invasive spatial information about the relative mechanical stiffness of the cell wall in a living plant and can be used for time-course studies.
Applied to the growing epidermal cells of the eudicot leaf, Altartouri et al. demonstrate the utility of this nascent laser-based technique for accessing new information about the cell wall during lobe formation. Using the Arabidopsis anisotropy1 mutant, shown to produce cellulose with less crystalline structure than native microfibrils (Fujita et al., 2013), Brillouin microscopy identifies regions of the cell wall with lower intrinsic stiffness than wild type, as expected for this mutant. The linearity of the measurement and the calibration from relative to absolute changes in material stiffness remain as important technical challenges. Much like any new technology, and especially when porting a laser-based spectroscopy to an optical scanning microscope, there will be new limitations to understand as well as opportunities for innovation. How the focused beam induces and reports the information about perturbations occurring mostly transverse to the beam is not well defined and the exact interpretation of total wall stiffness relative to wall thickness, composition, and the patterned deposition of cellulose will certainly be refined in time. The initial results from Altartouri et al. suggest that the reduction in periclinal wall stiffness observed in the anisotropy1 mutant has little effect on lobe initiation in the early leaf cells, but ultimately limits the full extension of the lobe during cell growth. Manipulating the chemical state of the pectic polysaccharides seems to have an opposite effect, suggesting that pectic polysaccharides are more critical for the initial formation of the lobes. The new data are used to propose a two-step model for lobe initiation and maturation that is certain to spark healthy and critical debate in a field hungry for new information about how cells expand to form the fantastic array of shapes observed in our natural world.
Acknowledgements
SLS would like to thank the Plant Physiology editors for their efforts toward this commentary and willingness to highlight new technologies. SLS wishes to thank the National Science Foundation for funding MCB:1615907.
LITERATURE CITED
Anderson CT, Carroll A, Akhmetova L, Somerville C (2010) Real-time imaging of cellulose reorientation during cell wall expansion in Arabidopsis roots. Plant Physiol 152: 787-796
Armour WJ, Barton DA, Law AM, Overall RL (2015) Differential Growth in Periclinal and Anticlinal Walls during Lobe Formation in Arabidopsis Cotyledon Pavement Cells. Plant Cell 27: 2484-2500
Baskin TI (2005) Anisotropic expansion of the plant cell wall. Annu Rev Cell Dev Biol 21: 203-222
Carpita NC, Gibeaut DM (1993) Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J 3: 1-30
Chen XY, Kim JY (2009) Callose synthesis in higher plants. Plant Signal Behav 4: 489-492
Cosgrove DJ (1998) Cell wall loosening by expansins. Plant Physiol 118: 333-339
Cosgrove DJ (2014) Re-constructing our models of cellulose and primary cell wall assembly. Curr Opin Plant Biol 22: 122-131
Cosgrove DJ (2016) Plant cell wall extensibility: connecting plant cell growth with cell wall structure, mechanics, and the action of wall-modifying enzymes. J Exp Bot 67: 463-476
Cox G, Moreno N, Feijo J (2005) Second-harmonic imaging of plant polysaccharides. J Biomed Opt 10: 024013
Dumais J, Shaw SL, Steele CR, Long SR, Ray PM (2006) An anisotropic-viscoplastic model of plant cell morphogenesis by tip growth. Int J Dev Biol 50: 209-222
Eichhorn S, Young R (2001) The Young’s modulus of a microcrystalline cellulose. Cellulose 8: 197-207
Elsayad K, Werner S, Gallemi M, Kong J, Sanchez Guajardo ER, Zhang L, Jaillais Y, Greb T, Belkhadir Y (2016) Mapping the subcellular mechanical properties of live cells in tissues with fluorescence emission-Brillouin imaging. Sci Signal 9: rs5
Elsner J, Lipowczan M, Kwiatkowska D (2018) Differential growth of pavement cells of Arabidopsis thaliana leaf epidermis as revealed by microbead labeling. Am J Bot 105: 257-265
Fu Y, Gu Y, Zheng Z, Wasteneys G, Yang Z (2005) Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell 120: 687-700
Fujita M, Himmelspach R, Ward J, Whittington A, Hasenbein N, Liu C, Truong TT, Galway ME, Mansfield SD, Hocart CH, Wasteneys GO (2013) The anisotropy1 D604N mutation in the Arabidopsis cellulose synthase1 catalytic domain reduces cell wall crystallinity and the velocity of cellulose synthase complexes. Plant Physiol 162: 74-85
Gendreau E, Traas J, Desnos T, Grandjean O, Caboche M, Hofte H (1997) Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol 114: 295-305
Gonneau M, Hofte H, Vernhettes S (2012) Fluorescent tags to explore cell wall structure and dynamics. Front Plant Sci 3: 145
Green PB (1962) Mechanism for Plant Cellular Morphogenesis. Science 138: 1404-1405
Higaki T, Takigawa-Imamura H, Akita K, Kutsuna N, Kobayashi R, Hasezawa S, Miura T (2017) Exogenous Cellulase Switches Cell Interdigitation to Cell Elongation in an RIC1-dependent Manner in Arabidopsis thaliana Cotyledon Pavement Cells. Plant Cell Physiol 58: 106-119
Majda M, Grones P, Sintorn IM, Vain T, Milani P, Krupinski P, Zagorska-Marek B, Viotti C, Jonsson H, Mellerowicz EJ, Hamant O, Robert S (2017) Mechanochemical Polarization of Contiguous Cell Walls Shapes Plant Pavement Cells. Dev Cell 43: 290-304 e294
Mansfield JC, Littlejohn GR, Seymour MP, Lind RJ, Perfect S, Moger J (2013) Label-free chemically specific imaging in planta with stimulated Raman scattering microscopy. Anal Chem 85: 5055-5063
McCann MC, Hammouri M, Wilson R, Belton P, Roberts K (1992) Fourier transform infrared microspectroscopy is a new way to look at plant cell walls. Plant Physiol 100: 1940-1947
Mccann MC, Roberts K (1994) Changes in Cell-Wall Architecture during Cell Elongation. Journal of Experimental Botany 45: 1683-1691
Micheli F (2001) Pectin methylesterases: cell wall enzymes with important roles in plant physiology. Trends Plant Sci 6: 414-419
Panteris E, Galatis B (2005) The morphogenesis of lobed plant cells in the mesophyll and epidermis: organization and distinct roles of cortical microtubules and actin filaments. New Phytol 167: 721-732
Paredez AR, Somerville CR, Ehrhardt DW (2006) Visualization of cellulose synthase demonstrates functional association with microtubules. Science 312: 1491-1495
Peaucelle A, Braybrook S, Hofte H (2012) Cell wall mechanics and growth control in plants: the role of pectins revisited. Front Plant Sci 3: 121
Peaucelle A, Wightman R, Hofte H (2015) The Control of Growth Symmetry Breaking in the Arabidopsis Hypocotyl. Curr Biol 25: 1746-1752
Sapala A, Runions A, Routier-Kierzkowska AL, Das Gupta M, Hong L, Hofhuis H, Verger S, Mosca G, Li CB, Hay A, Hamant O, Roeder AH, Tsiantis M, Prusinkiewicz P, Smith RS (2018) Why plants make puzzle cells, and how their shape emerges. Elife 7
Scarcelli G, Kim P, Yun SH (2011) In vivo measurement of age-related stiffening in the crystalline lens by Brillouin optical microscopy. Biophys J 101: 1539-1545
Scarcelli G, Polacheck WJ, Nia HT, Patel K, Grodzinsky AJ, Kamm RD, Yun SH (2015) Noncontact three-dimensional mapping of intracellular hydromechanical properties by Brillouin microscopy. Nat Methods 12: 1132-1134
Scarcelli G, Yun SH (2007) Confocal Brillouin microscopy for three-dimensional mechanical imaging. Nat Photonics 2: 39-43
Steeves TA, Sussex IM (1989) Patterns in plant development, Ed 2nd. Cambridge University Press, Cambridge England ; New York
Sussman M (2019) (Personal Commincation).
Szymanski DB (2014) The kinematics and mechanics of leaf expansion: new pieces to the Arabidopsis puzzle. Curr Opin Plant Biol 22: 141-148
Szymanski DB, Cosgrove DJ (2009) Dynamic coordination of cytoskeletal and cell wall systems during plant cell morphogenesis. Curr Biol 19: R800-811
Van Sandt VS, Suslov D, Verbelen JP, Vissenberg K (2007) Xyloglucan endotransglucosylase activity loosens a plant cell wall. Ann Bot 100: 1467-1473
Vofely RV, Gallagher J, Pisano GD, Bartlett M, Braybrook SA (2019) Of puzzles and pavements: a quantitative exploration of leaf epidermal cell shape. New Phytol 221: 540-552