Pyrethrin biosynthesis: From a phytohormone to specialized metabolite
Raimund Nagel
Universität Leipzig
Department of Plant Physiology
Leipzig, Saxony 04103
Germany
raimund.nagel@uni-leipzig.de
Dalmatian chrysanthemum (Tanacetum cinerariifolium) contains pyrethrins, which are highly effective natural insecticides that are nontoxic to most animals and biodegradable. Purification of these natural compounds, however, is more costly than production of their synthetic analogs, although the synthetic analogs are less biodegradable and can pose a danger for vertebrates. As we have become more environmentally conscious, the biosynthesis pathway of pyrethrins has attracted greater interest.
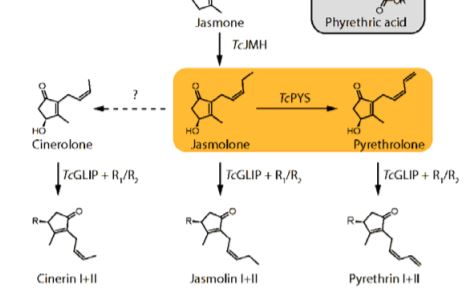
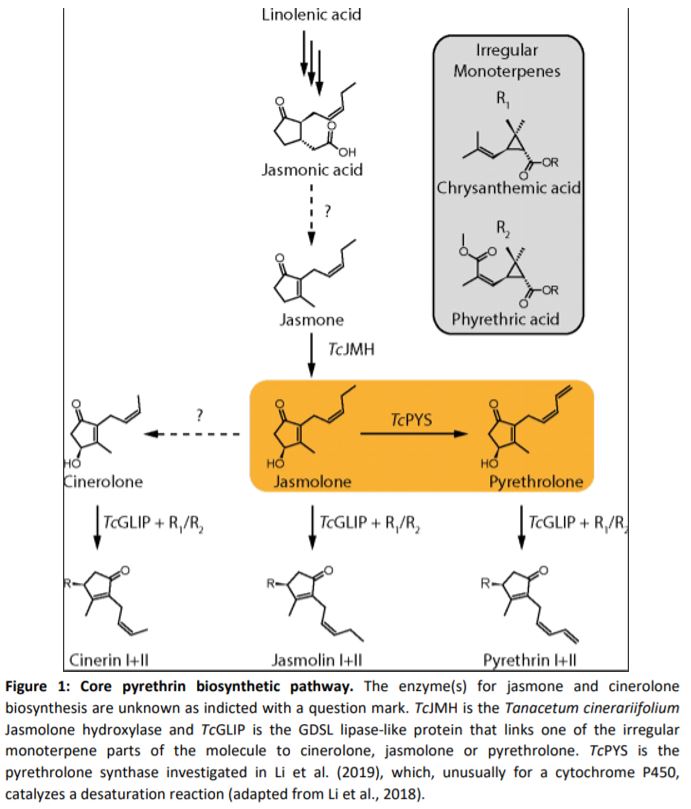 Pyrethrins are synthesized by the linking of two parts (Figure 1). The first is an irregular monoterpene whose biosynthesis is well established (Xu et al., 2019), and the second is derived from the phytohomone jasmonate. The biosynthesis pathway of jasmonates and their role as defensive hormones is well established (Wasternack and Hause, 2013), but less is known about how pyrethrins are synthesized. Recent publications have already elucidated some of the steps, including the production of jasmolone from jasmone by jasmone hydroxylase, but the enzyme responsible for the desaturation of the alkyl chain in jasmolone remains unknown (Li et al., 2018). This step is specifically important for the biosynthesis of Pyrethrin I and II, which are the most abundant compounds and namesakes for this class of compounds.
Pyrethrins are synthesized by the linking of two parts (Figure 1). The first is an irregular monoterpene whose biosynthesis is well established (Xu et al., 2019), and the second is derived from the phytohomone jasmonate. The biosynthesis pathway of jasmonates and their role as defensive hormones is well established (Wasternack and Hause, 2013), but less is known about how pyrethrins are synthesized. Recent publications have already elucidated some of the steps, including the production of jasmolone from jasmone by jasmone hydroxylase, but the enzyme responsible for the desaturation of the alkyl chain in jasmolone remains unknown (Li et al., 2018). This step is specifically important for the biosynthesis of Pyrethrin I and II, which are the most abundant compounds and namesakes for this class of compounds.
In this issue of Plant Physiology, Li et al. (2019) investigated this desaturation reaction. Previous expression analysis identified ten candidate enzymes, all belonging to the cytochrome P450 (CYP) superfamily (Xu et al., 2018). Most plants encode large numbers of CYPs in their genome, and these enzymes are known to catalyze a multitude of complex reactions that are essential for natural product biosynthesis (Hamberger and Bak, 2013). To identify the enzyme catalyzing jasmolone desaturation, candidates were transiently expressed together with TcJMH, the gene encoding jasmone hydroxylase, in Nicotiana benthamiana, with subsequent immersion in jasmone solution for substrate delivery. Only one CYP, which was henceforth named pyrethrolone synthase (TcPYS), was able to produce pyrethrolone. Further characterizations of TcPYS showed it to be highly substrate specific and localized to the ER membrane of trichomes on the ovaries of chrysanthemum flowers.
TcPYS, which is also called CYP82Q3 according to CYP nomenclature, is part of the CYP82 family. This CYP family is heavily involved in the biosynthesis of natural products from the flavonoid, alkaloid, terpene, and coumarin classes. The majority of characterized CYP82 enzymes catalyze hydroxylation reactions, which makes the desaturation catalyzed by TcPYS unusual. Moreover, only a very limited number of CYPs outside of the CYP82 family are known to catalyze such a reaction (Zhang and Li, 2017; Guengerich, 2018). Therefore, TcPYS is also an interesting target for investigations into the reaction mechanism of this desaturation reaction that can potentially progress through two different reaction pathways.
This publication identified a mechanistically interesting step in pyrethrin biosynthesis, which could ultimately lead to a less expensive synthetic biology route for production of these natural insecticides that are less ecotoxic than their synthetic analogs. However, for this to occur, the earlier biosynthetic steps from jasmonic acid, or potentially a precursor of jasmonic acid, must be identified.
This pathway is also intriguing as it has previously been shown that jasmonate hormones induce production of pyrethrins through transcriptional regulation of pyrethrin biosynthetic genes, and jasmonate also serves as their biosynthetic precursor. This observation raises questions about the complicated regulation of a highly abundant natural product that is derived from a significantly less abundant phytohormone. How is the balance maintained?
LITERATURE CITED
Guengerich FP (2018) Mechanisms of Cytochrome P450-Catalyzed Oxidations. ACS catalysis 8: 10964-10976
Hamberger B, Bak S (2013) Plant P450s as versatile drivers for evolution of species-specific chemical diversity. Philos Trans R Soc Lond B Biol Sci 368
Li W, Lybrand DB, Zhou F, Last RL, Pichersky E (2019) Pyrethrin biosynthesis: The cytochrome P450 oxidoreducatse CYP82Q3 converts jasmolone to pyrethrolone. Plant Physiol DOI: https://doi.org/10.1104/pp.19.00499
Li W, Zhou F, Pichersky E (2018) Jasmone Hydroxylase, a Key Enzyme in the Synthesis of the Alcohol Moiety of Pyrethrin Insecticides. Plant Physiology 177: 1498-1509
Wasternack C, Hause B (2013) Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Annals of Botany 111: 1021-1058
Xu H, Li W, Schilmiller AL, van Eekelen H, de Vos RCH, Jongsma MA, Pichersky E (2019) Pyrethric acid of natural pyrethrin insecticide: complete pathway elucidation and reconstitution in Nicotiana benthamiana. New Phytologist 223: 751-765
Xu H, Moghe GD, Wiegert-Rininger K, Schilmiller AL, Barry CS, Last RL, Pichersky E (2018) Coexpression Analysis Identifies Two Oxidoreductases Involved in the Biosynthesis of the Monoterpene Acid Moiety of Natural Pyrethrin Insecticides in Tanacetum cinerariifolium. Plant Physiol 176: 524-537
Zhang X, Li S (2017) Expansion of chemical space for natural products by uncommon P450 reactions. Natural Product Reports