Moonlighting Role of Plastidial NAD-MDH
Schreier et al. investigate the role of a plastid malate dehydrogenase in early chloroplast development. Plant Cell
Background: Malate dehydrogenases (MDH) are enzymes that are widespread in prokaryotes and eukaryotes. MDH interconverts oxaloacetate and malate, using either NAD or NADP as a cofactor. In plant cells, forms of MDH are found in several subcellular compartments. There are two MDHs in chloroplasts – one uses NADP, while the other uses NAD (called pdNAD-MDH). It was proposed that their activities could help to maintain redox homeostasis in the chloroplast during the day and night, respectively. Mutations in Arabidopsis that abolish NADP-dependent MDH have a minor impact on plant growth. However, the loss of pdNAD-MDH is embryo lethal, showing that it is an essential protein.
Question: We aimed to understand the role of pdNAD-MDH and discover what makes it so important during embryo development. This question is important, since pdNAD-MDH is the only form of MDH whose loss causes embryo-lethality and because this mutant phenotype cannot be explained at the biochemical level.
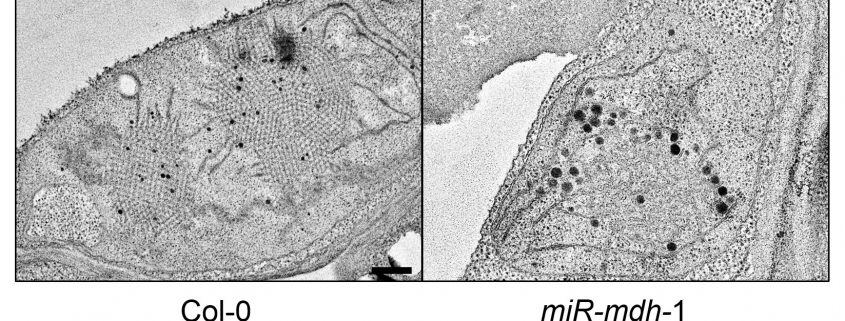
Findings: Using a range of approaches, we showed that pdNAD-MDH is needed for the proper development of chloroplasts, which is essential during embryogenesis. Surprisingly, we found that the enzymatic activity of pdNAD-MDH is not required in these processes, but only the pdNAD-MDH protein itself. This was shown by replacing the endogenous active pdNAD-MDH protein with inactive versions – the resulting plants grew like the wild type. This led us to discover a second, moonlighting function of pdNAD-MDH, distinct from its enzymatic activity. We found that pdNAD-MDH interacts with a protein complex at the chloroplast inner envelope membrane. This protein complex is composed of ATP-dependent proteases: FtsH12 and its inactive subunits FtsHi. This complex, like pdNAD-MDH, is essential for chloroplast development and embryogenesis. Interestingly, repressing pdNAD-MDH also reduces the abundance of the FtsH12 protein. Therefore, we propose that the pdNAD-MDH protein plays an essential moonlighting role in stabilizing the FtsH12 complex.
Next steps: Future studies will need to focus on establishing the generality of what we have shown in Arabidopsis and on determining the precise molecular function of the FtsH12-FtsHi complex. The biological significance of the recruitment of this widespread metabolic enzyme for this specific stabilizing role needs to be discovered.
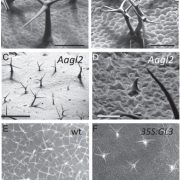
Figure: Etioplast ultrastructure in cotyledons of etiolated Col-0 wild-type and miR-mdh-1 mutant seedlings observed by transmission electron microscopy.
Tina B. Schreier, Antoine Cléry, Michael Schläfli, Florian Galbier, Martha Stadler, Emilie Demarsy, Daniele Albertini, Benjamin A. Maier, Felix Kessler, Stefan Hörtensteiner, Samuel C. Zeeman, Oliver Kötting. (2018). Plastidial NAD-Dependent Malate Dehydrogenase: A Moonlighting Protein Involved in Early Chloroplast Development through Its Interaction with an FtsH12-FtsHi Protease Complex. Plant Cell 30: 1745-1769; DOI: https://doi.org/10.1105/tpc.18.00121