Keep sugar away to stay active: glycosylation of methyl salicylate shuts down systemic signaling
Plant hormones are frequently modified by glycosylation, hydroxylation, methylation and other conjugations, and these modifications can alter the hormone’s activity and stability (Wang et al., 2019). The phytohormone salicylic acid orchestrates effective defense and mediates local and systemic-acquired resistance (SAR) against biotrophic pathogens such as the bacterium Pseudomonas syringae. Reversible glucose conjugation of salicylic acid aids its storage and formation of methyl ester salicylic acid (MeSA) facilitates long-distance transport and systemic communication (Park et al., 2007). Mounting a competent defense response is, therefore, critically dependent on the homeostasis between active salicylic acid molecules and inactive conjugated forms as well as movement of mobile signals. Many factors are thought to influence this homeostasis (Lui et al., 2011).
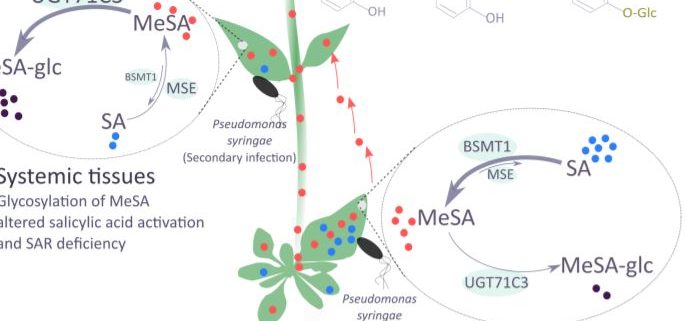
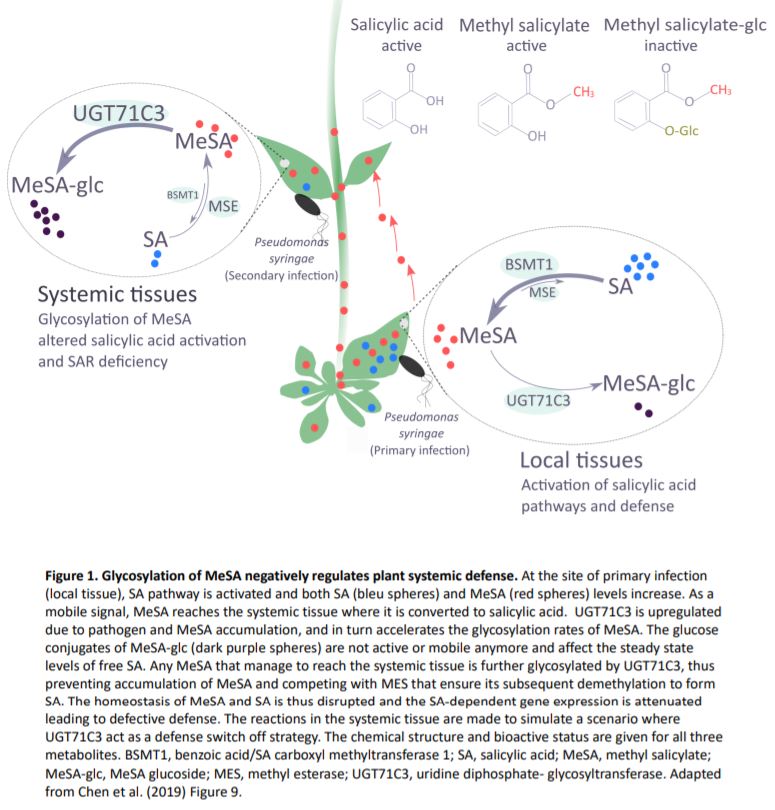 In this issue of Plant Physiology, Chen et al. (2019) studied the impact of glycosylation of MeSA on the homeostasis of salicylic acid, and hence, on plant immunity. The authors identified the occurrence of glucose conjugates of MeSA and presented evidence for a key role of one particular uridine diphosphate- glycosyltransferase (UGT) in modulating SAR in Arabidopsis (Arabidopsis thaliana). Upon infection with Pseudomonas, UGT71C3 is induced at the transcriptional level mainly in leaves, pointing to a potential role in immunity. In vivo and in vitro biochemical analyses showed that UGT71C3 acts specifically on methyl salicylate, but not salicylic acid itself or other structurally close benzoic acids.
In this issue of Plant Physiology, Chen et al. (2019) studied the impact of glycosylation of MeSA on the homeostasis of salicylic acid, and hence, on plant immunity. The authors identified the occurrence of glucose conjugates of MeSA and presented evidence for a key role of one particular uridine diphosphate- glycosyltransferase (UGT) in modulating SAR in Arabidopsis (Arabidopsis thaliana). Upon infection with Pseudomonas, UGT71C3 is induced at the transcriptional level mainly in leaves, pointing to a potential role in immunity. In vivo and in vitro biochemical analyses showed that UGT71C3 acts specifically on methyl salicylate, but not salicylic acid itself or other structurally close benzoic acids.
SAR occurs with the onset of salicylic acid signaling in the local infected tissue and the activation of defense in the distal tissues that have not been exposed to the pathogen. Chen and coworkers show that UGT71C3 overexpression leads to more severe disease symptoms and SAR deficiency. Conversely, the loss-of-function mutants showed substantially reduced symptoms and accumulated more salicylic acid and MeSA compared to wild-type plants. These phenotypes are associated with differential expression of SAR-related genes. The activation of SAR requires optimal communication between the local and the systemic leaves and accumulation of both MeSA and salicylic acid to adequate levels. The authors demonstrate that glycosylation of MeSA alters the homeostasis of MeSA and salicylic and prevents further conversion of MeSA to salicylic acid, which is required for proper development of SAR. Collectively, the data presented by Chen et al. establishes UGT71C3 as a key and novel component of plant immunity negatively modulating the MeSA-dependent SAR (Figure 1).
Mobile signals other than MeSA have been described as SAR signals, including chemically diverse molecules like the lipid transfer protein DEFECTIVE IN INDUCED RESISTANCE1, azelaic acid and the lysine derivative pipecolic acid (Dempsey and Klessig, 2012; Hartmann and Zeier, 2019). Chen et al., 2019 showed that an additional layer of regulation can be added to this sophisticated and complex network. So, one could ask how do plants manage that many SAR signals and how are these connected and perceived in the systemic tissue to further activate gene expression and de novo salicylic acid biosynthesis. On the other hand, how do plants fine-tune SAR and switch it off to avoid excessive costs, once a dynamic and efficient response is in place. Addressing these questions will enhance our understanding of the mechanisms to achieve SAR. For the time being, this new report shows that glycosylation of immune-related compounds is an important factor.
Analysis of plant UGTs continues to attract interest and the role of previously uncharacterized members of this big family is emerging (Song et al., 2016; Thompson et al., 2017; Huang et al., 2018; Irmisch et al., 2019). Investigating UGT functions is enabled and facilitated by the availability of plant genome sequences and the development of large-scale methods for data-gathering and analysis. A key question is how many glycosyltransferases remain to be characterized. The answer is, too many to handle if we continue with the strategy of analyzing single genes. Scientific breakthroughs in this field can be achieved by using approaches that couple functional and structural analyses to data-driven machine learning for prediction of enzyme activity (Yang et al., 2018).
Amna Mhamdi, Ghent University
References
Chen L, Wang W-S, Wang T, Meng X-F, Chen T-T, Huang X-X, Li Y-J, Hou B-K (2019). Methyl salicylate glucosylation regulates plant defense signalling and systemic acquired resistance. Plant Physiol. DOI: 10.1104/pp.19.00091
Dempsey DA, Klessig DF (2012). SOS-too many signals for systemic acquired resistance? Trends Plant Sci. 17, 538-545
Hartmann M, Zeier J (2019). N-hydroxypipecolic acid and salicylic acid: a metabolic duo for systemic acquired resistance. Curr. Opin. Plant Biol. 50, 44-57
Huang X, Zhu GQ, Liu Q, Chen L, Li YJ, Hou BK (2018). Modulation of plant salicylic acid-associated immune responses via glycosylation of dihydroxybenzoic acids. Plant Physiol. 176, 3103-3119
Irmisch S, Ruebsam H, Jancsik S, Yuen MMS, Madilao L, Bohlmann J (2019). Synthetic biology of montbretin A biosynthesis. Plant Physiol. DOI: 10.1104/pp.19.00254
Liu PP, von Dahl CC, Klessig DF (2011). The extent to which methyl salicylate is required for signaling systemic acquired resistance is dependent on exposure to light after infection. Plant Physiol. 157, 2216-2226
Park SW, Kaimoyo E, Kumar D, Mosher S, Klessig DF (2007). Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 318, 887 113-116
Song C, Hong X, Zhao S, Liu J, Schulenburg K, Huang F-C, Franz-Oberdorf K, Schwab W (2016). Glucosylation of 4-hydroxy-2,5-dimethyl-3(2H)-furanone, the key strawberry flavor compound in strawberry fruit. Plant Physiol. 171, 139-151
Thompson, AMG, Iancu, CV, Neet, KE, Dean, JV, Choe, J-Y (2017) Differences in salicylic acid glucose conjugation by UGT74F1 and UGT74F2 from Arabidopsis thaliana. Sci. Rep. 7, 46629.
Wang S, Alseekh S, Fernie AR, Luo J (2019). The structure and function of major plant metabolite modifications. Mol. Plant. doi: https://doi.org/10.1016/j.molp.2019.06.001.
Yang M, Fehl C, Lees KV, Lim E-K, Offen WA, Davies GJ, Bowles DJ, Davidson MG, Roberts SJ, Davis BG (2018). Functional and informatics analysis enables glycosyltransferase activity prediction. Nat. Chem. Biol. 14, 1109-1117