Developmental timing is everything (part II): gating of high temperature responses by the circadian clock
 Arabidopsis thaliana seedlings exposed to heat stress (>42ºC) suffer high lethality rates unless exposed to milder heat (37ºC) beforehand. This pre-exposure distinguishes basal from acquired thermotolerance and has been extensively studied over the years. However, experimental conditions used to study responses to elevated temperatures do not always adhere to a standard protocol and can vary in the duration, intensity, and timing of heat treatment. These factors potentially contribute to increasing experimental noise. In their article, Li and colleagues (2019) explain why the timing of the heat stress matters. Here is a hint: the clock genes REVEILLE 4 and 8 (RVE4/8) play an essential role.
Arabidopsis thaliana seedlings exposed to heat stress (>42ºC) suffer high lethality rates unless exposed to milder heat (37ºC) beforehand. This pre-exposure distinguishes basal from acquired thermotolerance and has been extensively studied over the years. However, experimental conditions used to study responses to elevated temperatures do not always adhere to a standard protocol and can vary in the duration, intensity, and timing of heat treatment. These factors potentially contribute to increasing experimental noise. In their article, Li and colleagues (2019) explain why the timing of the heat stress matters. Here is a hint: the clock genes REVEILLE 4 and 8 (RVE4/8) play an essential role.
The authors reasoned that responses to high temperatures should be rapid and therefore conducted transcriptome sequencing of seedlings and adult plants during the first 30 minutes of exposure to 37ºC. mRNA levels exhibit significant changes within 150 seconds that are initially dominated by the down-regulation of hundreds of genes. The first wave of gene expression activation begins within 10 minutes and initiates subsequent cascades, observed over the next 20 minutes.
Assuming a transcriptional basis, the promoters of all induced genes should bear the mark of their upstream regulators. This hypothesis prompted the authors to itemize all cis-regulatory elements: not surprisingly, about 60% of promoters from induced genes contain binding sites for Heat Shock Factors (HSFs). A quadruple mutant inactivating all four HSFA1 sub-family genes provided the genetic validation of these observations: the mutant no longer induces the expression of HSF-dependent genes, as expected. Both basal and acquired thermotolerance were greatly attenuated in the quadruple mutant.
But what about the remaining 40% of genes whose promoters lack binding sites for HSFs? These genes are, by definition, HSF-independent, retain full expression in the hsfa1 quadruple mutant after heat shock treatment, and thus define a second transcription regulatory pathway. Only one DNA-binding motif is unique to their promoters: the so-called Evening Element (EE), recognized by the MYB-domain transcription factors RVE4 and RVE8 from the REVEILLE family. The timing of heat treatment (3 to 6 hours after lights on) overlaps satisfyingly with the window of maximal RVE4 and RVE8 protein accumulation.
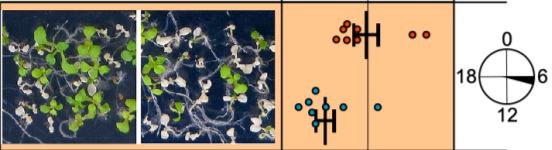
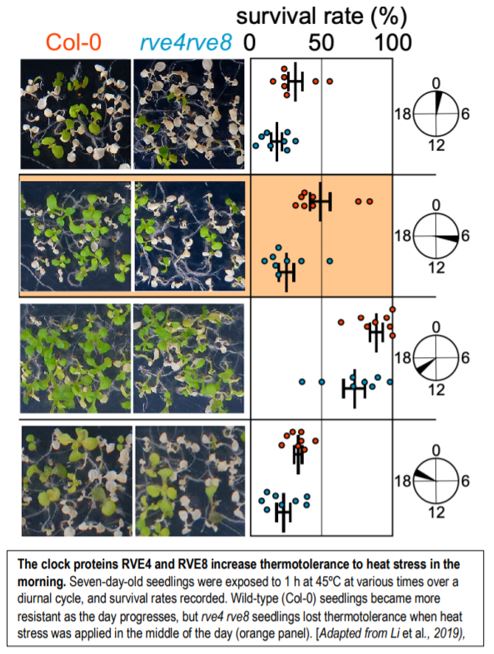
If RVE4/8 play a role in the establishment of thermotolerance, then the mutant should be more sensitive to heat stress. That is indeed the case, but this depends on the time of day: the rve4 rve8 double mutant is more susceptible to heat when it is applied mid-morning (see Figure), although it remains more resistant than the hsfa1 quadruple mutant. To close the loop, the authors went on to demonstrate that RVE4/8 mediate thermotolerance, at least partially, by inducing the expression of two ETHYLENE RESPONSE FACTOR genes, ERF53 and ERF54, via the EE found within their promoters. ERF53 and ERF54 expression is responsive to heat stress in wild type and the hsfa1 quadruple mutant, but not in rve4 rve8, consistent with their position downstream of RVE4/8, and parallel to HSFA1s. Fittingly, overexpression of ERF53 enhances thermotolerance (Hsieh et al., 2013).
These results, therefore, provide a simple framework for the coordination (or gating) of temperature responses by the circadian clock. Mild heat stress in the morning activates the transcription factors HSFA1s and RVE4/8, in turn inducing the expression of their respective downstream targets, among which other HSFs and ERF53/54 feature prominently. Future questions to decipher include the post-transcriptional mechanisms activating HSFA1s and RVE4/8. Besides, plant responses to heat stress vary during the day, and the authors hypothesize the involvement of another clock gene that modulates thermotolerance later in the day.
Patrice A. Salomé
Department of Chemistry and Biochemistry
University of California, Los Angeles
salome@chem.ucla.edu
References:
Hsieh EJ, Cheng MC and Lin TP (2013). Functional characterization of an abiotic stress-inducible transcription factor AtERF53 in Arabidopsis thaliana. Plant Mol. Biol. 82: 223-237.
Li B, Gao Z, Liu X, Sun D-Y and Tang W (2019). REV4/8 Control Heat Shock-induced Gene Expression. The Plant Cell, Published July 2019. DOI: https://doi.org/10.1105/tpc.00519.2019