Combinatorial Chemistry Solutions to Explore Intracellular Trafficking
Mishev, Lu, et al. discover a new chemical inhibitor of a protein family involved in the regulation of endomembrane transport. Mishev, Lu, et al. Plant Cell (2018) https://doi.org/10.1105/tpc.18.00145
By Kiril Mishev and Qing Lu
Background: The structural and functional compartmentalization in eukaryotic cells allows the orchestration of highly coordinated cellular processes that rely on the continuous exchange of molecular components within the cytoplasm and at the cell surface. The intracellular trafficking logistics comprises membrane-delimited organelles and numerous protein regulators that ensure cargo sorting, vesicle formation and targeting to the acceptor membrane. The small GTPases from the ARF protein family and their guanine exchange factors (GEFs) are such regulators with an essential role in membrane vesicle formation. The Arabidopsis genome encodes eight members of the ARF GEF family with overlapping functions in the intracellular transport which hampers their study by classical genetic approaches. Alternatively, the use of small-molecule inhibitors has turned out to be crucial for a better understanding of the ARF GEF-mediated endomembrane trafficking.
Question: Out of the pharmacological toolbox for ARF GEF inhibition, the fungal toxin Brefeldin A (BFA) is the only small molecule with proven bioactivity and known mode of action in plant cells. Our goal was to discover a new chemical tool for interference with ARF GEF-dependent processes that cannot be manipulated with BFA.
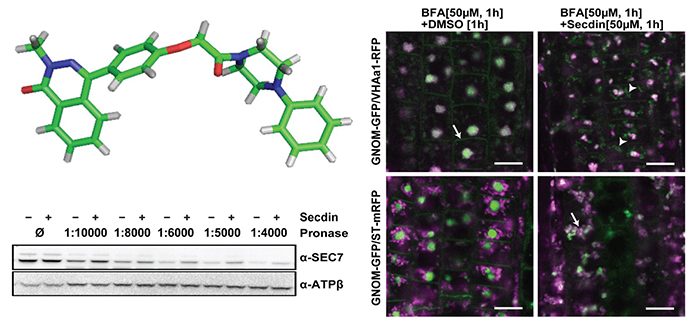
Findings: We used a chemical genetic approach to identify Secdin, a compound that impairs protein recycling, secretion, and endocytosis, and leads to the retention of plasma membrane proteins in late endosomal compartments in Arabidopsis cells. We demonstrated that, in contrast to BFA, Secdin targets all tested Arabidopsis ARF GEF proteins. We also found that the interaction of Secdin with the ARF GEFs does not involve the BFA-targeted catalytic domain. The different mode of action of the two ARF GEF inhibitors determines the unique vesicle trafficking phenotypes after single or combined treatment. Interestingly, we established that Secdin is bioactive in human cells as well.
Next steps: We would like to go further with the mode-of-action studies and test whether Secdin binds to a regulatory ARF GEF domain, thus impairing the interactions with other regulatory proteins that are important for ARF GEF recruitment to endomembranes. We also want to study the molecular targets of our compound in mammalian systems.
Mishev, Lu, et al. (2018). Nonselective Chemical Inhibition of Sec7 Domain-containing ARF GTPase Exchange Factors. Plant Cell 30: 2573-2593. https://doi.org/10.1105/tpc.18.00145
Keywords: chemical inhibitor, endomembrane trafficking, ARF GEFs