Ion channels regulate nitrogen–potassium balance
By Beibei Liu and Kai He
Ministry of Education Key Laboratory of Cell Activities and Stress Adaptations, School of Life Sciences, Lanzhou University, Lanzhou 730000, China
Background: The nitrogen (N)–potassium (K) balance not only profoundly affects plant growth but also serves as an essential signal to regulate plant–environment interactions. However, the detailed molecular mechanisms that maintain the N–K balance are largely unknown.
Question: How does the nitrate efflux channel SLAH3 regulate the N–K balance in plants?
Findings: The anion channel SLAH3 mediates NO3− efflux under high K+ conditions. The accelerated depolarization next causes the opening of the K+ channels GORK and SKOR to mediate K+ efflux. The channel–channel interaction may also be important for manipulating K+ channel activities. We found that the N–K balance is regulated by N and K channels, with both the membrane potential and the interactions between these membrane proteins modulating this regulation.
Next steps: As anion and cation channels function coordinately to regulate the N–K balance, the corresponding genes could be used to construct novel crop variants that can withstand diverse N–K environments through molecular breeding.
Beibei Liu, Changxin Feng, Xianming Fang, Zhen Ma, Chengbin Xiao, Shuaishuai Zhang, Zhenzhen Liu, Doudou Sun, Hongyong Shi, Xiaoqin Ding, Chenyang, Qiu, Jia Li, Sheng Luan, Legong Li, and Kai He. (2023). The anion channel SLAH3 interacts with potassium channels to regulate nitrogen–potassium homeostasis and the membrane potential in Arabidopsis. https://doi.org/10.1093/plcell/koad014


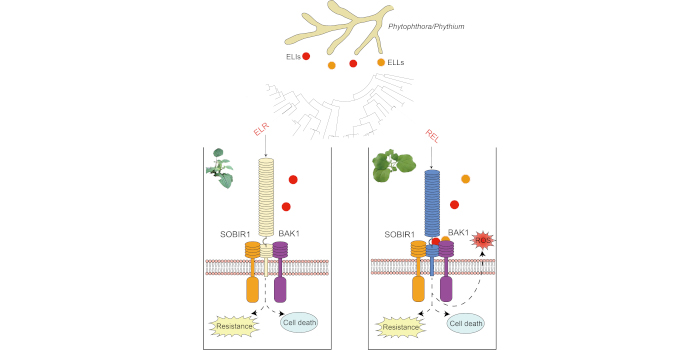

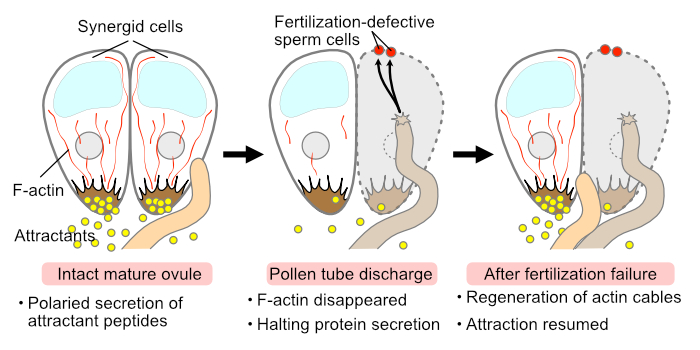 Findings: We demonstrated that microtubule destruction compromises the elongation of plasma membrane invaginations in the filiform apparatus. Disruption of F-actin resulted in severe disorganization of synergid cell morphology, causing incomplete filiform apparatus formation and aberrant positioning of the central vacuole. Furthermore, F-actin destruction impaired the secretion of pollen tube attractant peptides and caused female sterility. After pollen tube discharge, the longitudinal F-actin pattern temporarily disappeared in the persistent synergid. Our data suggest that F-actin has a central role in maintaining cell polarity and in mediating male–female communication in the synergid cell.
Findings: We demonstrated that microtubule destruction compromises the elongation of plasma membrane invaginations in the filiform apparatus. Disruption of F-actin resulted in severe disorganization of synergid cell morphology, causing incomplete filiform apparatus formation and aberrant positioning of the central vacuole. Furthermore, F-actin destruction impaired the secretion of pollen tube attractant peptides and caused female sterility. After pollen tube discharge, the longitudinal F-actin pattern temporarily disappeared in the persistent synergid. Our data suggest that F-actin has a central role in maintaining cell polarity and in mediating male–female communication in the synergid cell.
 Peng Li, first author of
Peng Li, first author of 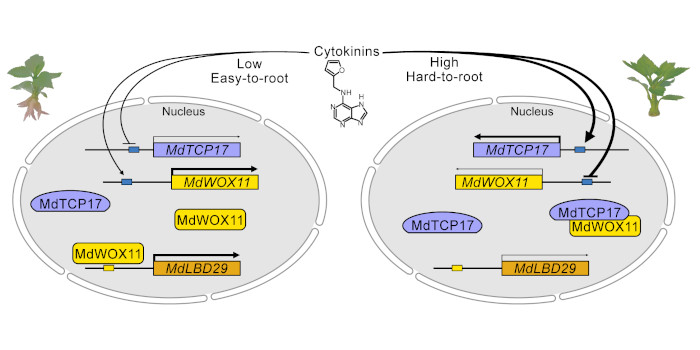 Findings: We found that a low endogenous cytokinin content improved AR formation in apples. We also found a negative correlation between the expression of cytokinin-responsive MdTCP17 and AR formation, while overexpression of MdTCP17 in transgenic apples inhibited AR formation. MdWOX11 promotes AR formation and MdTCP17 interacts with MdWOX11. MdWOX11 directly binds to the promoter of MdLBD29 and positively regulates its expression. Furthermore, MdTCP17 reduced the binding of MdWOX11 to the MdLBD29 promoter, and co-expression of MdTCP17 and MdWOX11 reduced MdLBD29 expression. Our study thus revealed a mechanism by which MdTCP17 and cytokinin inhibit AR primordium formation.
Findings: We found that a low endogenous cytokinin content improved AR formation in apples. We also found a negative correlation between the expression of cytokinin-responsive MdTCP17 and AR formation, while overexpression of MdTCP17 in transgenic apples inhibited AR formation. MdWOX11 promotes AR formation and MdTCP17 interacts with MdWOX11. MdWOX11 directly binds to the promoter of MdLBD29 and positively regulates its expression. Furthermore, MdTCP17 reduced the binding of MdWOX11 to the MdLBD29 promoter, and co-expression of MdTCP17 and MdWOX11 reduced MdLBD29 expression. Our study thus revealed a mechanism by which MdTCP17 and cytokinin inhibit AR primordium formation. Mazen Alazem, first author of
Mazen Alazem, first author of  Markéta Šámalová, first author of
Markéta Šámalová, first author of  Zhenkun Liao, first author of
Zhenkun Liao, first author of