Subcellular Spice Trade Routes: Crocin Biosynthesis in the Saffron Crocus (Crocus sativus)
Commentary by
Saffron is produced from the stigmas and styles of Crocus sativus flowers and is one of the most expensive spices. In C. sativus, both stigma and style are intensely crimson red in color due to the presence of three major classes of apocarotenoids: crocins, picrocrocin, and safranal. In addition to conferring color, these apocarotenoids—along with about 150 other minor components—give saffron its characteristic flavor, which is associated with traditional dishes such as zarda from Iran, paella from Spain, and bouillabaisse from France.
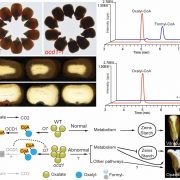
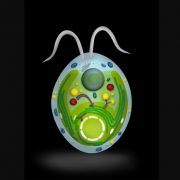
The general biosynthesis route for the apocarotenoids is known (Fig. 1), but most of the individual enzymes have not yet been identified in C. sativus. Zeaxanthin, the common precursor for all three classes, is cleaved by carotenoid cleavage dioxygenase 2 (CCD2) into 3-OH-β-cyclocitral and crocetin dialdehyde. 3-OH-β-cyclocitral is then converted in a multistep reaction to safranal, the major aroma volatile of saffron. Crocetin dialdehyde is first oxidized to crocetin and subsequently glycosylated to form crocins. In contrast to earlier intermediates, crocins are soluble in the aqueous environment of the vacuole, where they ultimately accumulate.
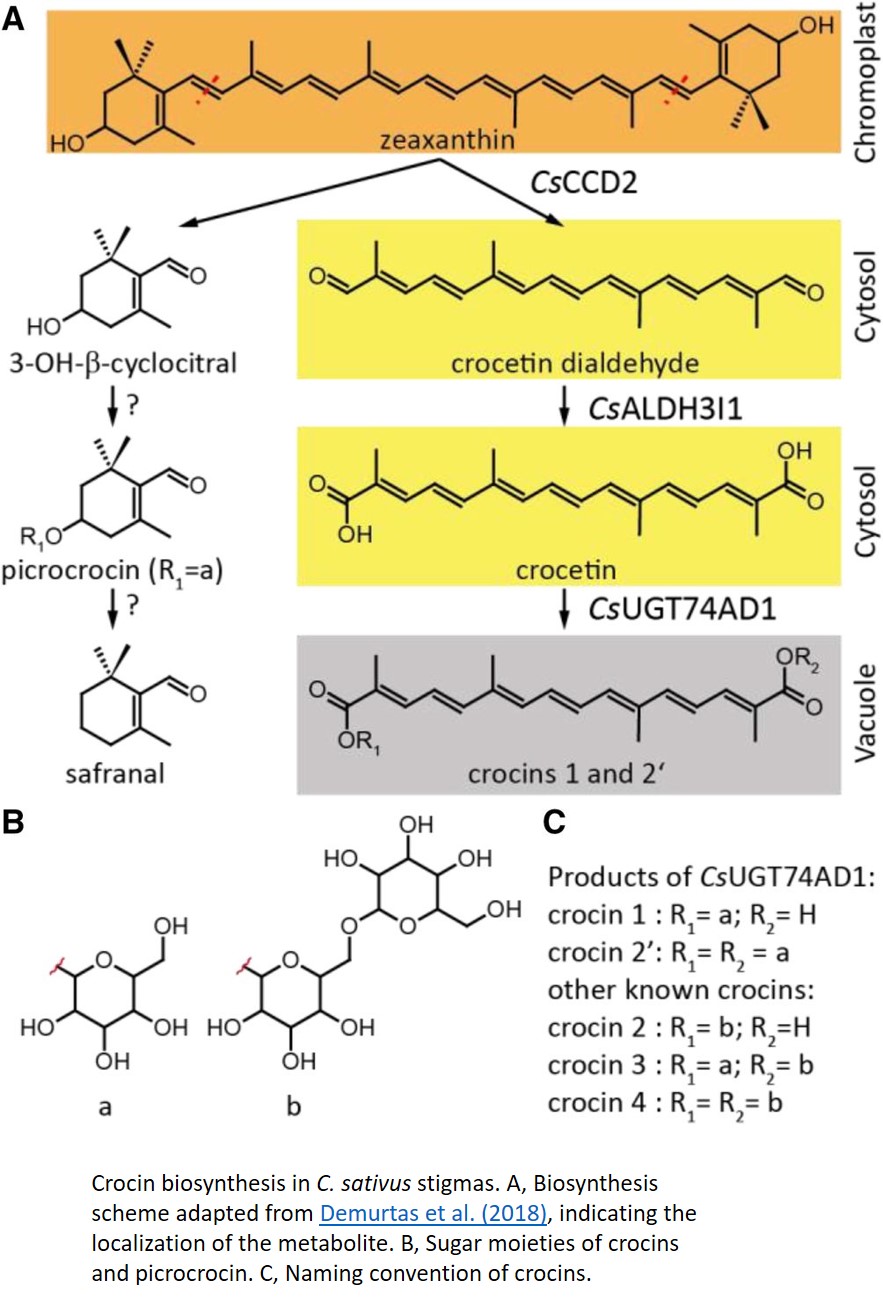
In this issue of Plant Physiology, Demurtas et al. (2018) describe the identification two enzymes in the crocin biosynthesis pathway. Biochemical characterization of the enzyme ALDH3I1 demonstrates that it catalyzes the oxidation of crocetin dialdehyde to form crocetin, and the glycosyl transferase UGT74AD1 adds the first sugars, producing crocins 1 and 2′.
While the biochemical identification of these enzymes is an important step in understanding the crocin biosynthesis pathway, an intriguing conclusion of this paper comes from the detailed investigation of the subcellular location of individual enzymes in this pathway. CCD2 is localized to the interior of the chromoplast, while ALDH3I1 is localized to the endoplasmic reticulum (ER). Both enzymes are predicted to have a transmembrane helix, which would explain their partitioning into insoluble and membranous subcellular fractions. UGT74AD1, by contrast, was shown to accumulate in the cytosol, where it associated with the cytoskeleton or membrane structures, despite its apparent lack of a transmembrane domain.
In recent years, large complexes of biosynthetic enzymes, termed metabolons, have been shown to be important for efficient shuttling of substrate intermediates through various pathways. This is especially true for secondary metabolites, which can represent a major fraction of cellular metabolism in specific cells and tissues (Laursen et al., 2015). However, in the case of crocins, which can constitute approximately 10% of the dry weight of saffron stigmas, Demurtas et al. (2018) have shown that biosynthesis is spread across at least three subcellular locations: chromoplasts, the ER surface, and the cytosol. This may represent an intriguing example of the ER membrane functioning as a hydrophobic highway that connects initial synthesis of lipid-soluble intermediates in the chromoplast, followed by the formation of water-soluble glycosylated products that ultimately accumulate in the vacuole. The authors highlight a similar scenario in the case of stevioside biosynthesis in Stevia rebaudiana leaves (Brandle and Telmer, 2007), suggesting that this may represent a more general mechanism for efficient subcellular trafficking of secondary metabolites.
REFERENCES
Brandle JE, Telmer PG (2007) Steviol glycoside biosynthesis. Phytochemistry 68: 1855–1863
Laursen T, Møller BL, Bassard J-E (2015) Plasticity of specialized metabolism as mediated by dynamic metabolons. Trends Plant Sci 20: 20–32