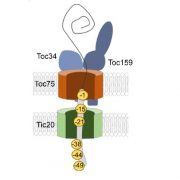
Mechanism of enzyme repair by the AAA+ chaperone Rubisco activase ($)
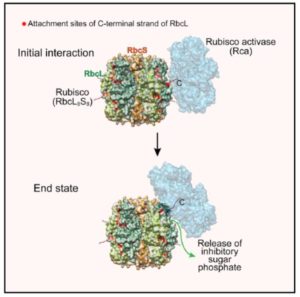 Rubisco is a fascinating enzyme, which in plants is a hexadecamer made up of eight large (RbcL) and eight small (RbcS) subunits. The catalytic sites are buried within the enzyme at the interfaces between pairs of RbcL subunits. Rubisco catalyzes the first step in the carbon-fixing photosynthetic reactions, but it does so inefficiently and with a tendency to make mistakes. One of its accidental products, xylulose-1,5-bisphosphate (XuBP), binds tightly to the active site until it is removed by another enzyme, Rubisco activase (Rca). Bhat et al. investigated the mechanism by which Rca repairs Rubisco. Through hydrogen/deuterium exchange, chemical crosslinking, and Cryo-EM, they found that Rca is able to remove the inhibitors with remarkably little disturbance to the overall structure of the enzyme. (I can’t help but think of my mother carefully unpicking and correcting a mistake in her knitting while reading this paper). Mol. Cell. 0.1016/j.molcel.2017.07.004
Rubisco is a fascinating enzyme, which in plants is a hexadecamer made up of eight large (RbcL) and eight small (RbcS) subunits. The catalytic sites are buried within the enzyme at the interfaces between pairs of RbcL subunits. Rubisco catalyzes the first step in the carbon-fixing photosynthetic reactions, but it does so inefficiently and with a tendency to make mistakes. One of its accidental products, xylulose-1,5-bisphosphate (XuBP), binds tightly to the active site until it is removed by another enzyme, Rubisco activase (Rca). Bhat et al. investigated the mechanism by which Rca repairs Rubisco. Through hydrogen/deuterium exchange, chemical crosslinking, and Cryo-EM, they found that Rca is able to remove the inhibitors with remarkably little disturbance to the overall structure of the enzyme. (I can’t help but think of my mother carefully unpicking and correcting a mistake in her knitting while reading this paper). Mol. Cell. 0.1016/j.molcel.2017.07.004





Leave a Reply
Want to join the discussion?Feel free to contribute!