Developmental Timing is Everything: TZP and Phytochrome Signaling
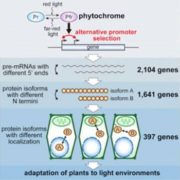
Although they lack eyes, plants can differentiate between colors with a full complement of photoreceptors. Phytochromes (phys) are such photoreceptors dedicated to the visible light spectrum ranging from red light (600-700 nm) to far-red light (700-750 nm). phys are some of the most studied genes and control plant architecture and flowering time. phyA is a special case: it mediates far-red rather than red light responses. Since leaves absorb red light but let most far-red light through, far-red light lets plant know they are growing under a canopy and may need to promote vertical growth to escape shade.
Despite decades of research, the phyA signaling cascade remains incomplete. Now, Zhang and colleagues (Zhang et al., 2018) show that the gene TANDEM ZINC-FINGER/PLUS3 (TZP) is important for phyA phosphorylation and full activation in far-red light.
TZP was initially identified as a major-effect quantitative trait locus in hypocotyl elongation (Loudet et al., 2008). The predicted loss-of-function natural allele resulted in shorter hypocotyls in blue and white light, while overexpression of TZP led to hypocotyl and petiole elongation. TZP gene expression is under the control of the circadian clock, with a peak at dawn, and most misexpressed genes in TZP overexpression lines similarly show peak expression at dawn (Loudet et al., 2008).
TZP encodes a nuclear protein that is very quickly recruited to nuclear photobodies (NBs) upon exposure to red light. This requires phyB, a light-stable phytochrome that perceives red light. TZP does not move to NBs in response to far-red light, nor do phyA mutants prevent NB formation in white light. NBs mark sites of active transcription, as they disappear when seedlings are treated with transcription inhibitors (Kaiserli et al., 2015).
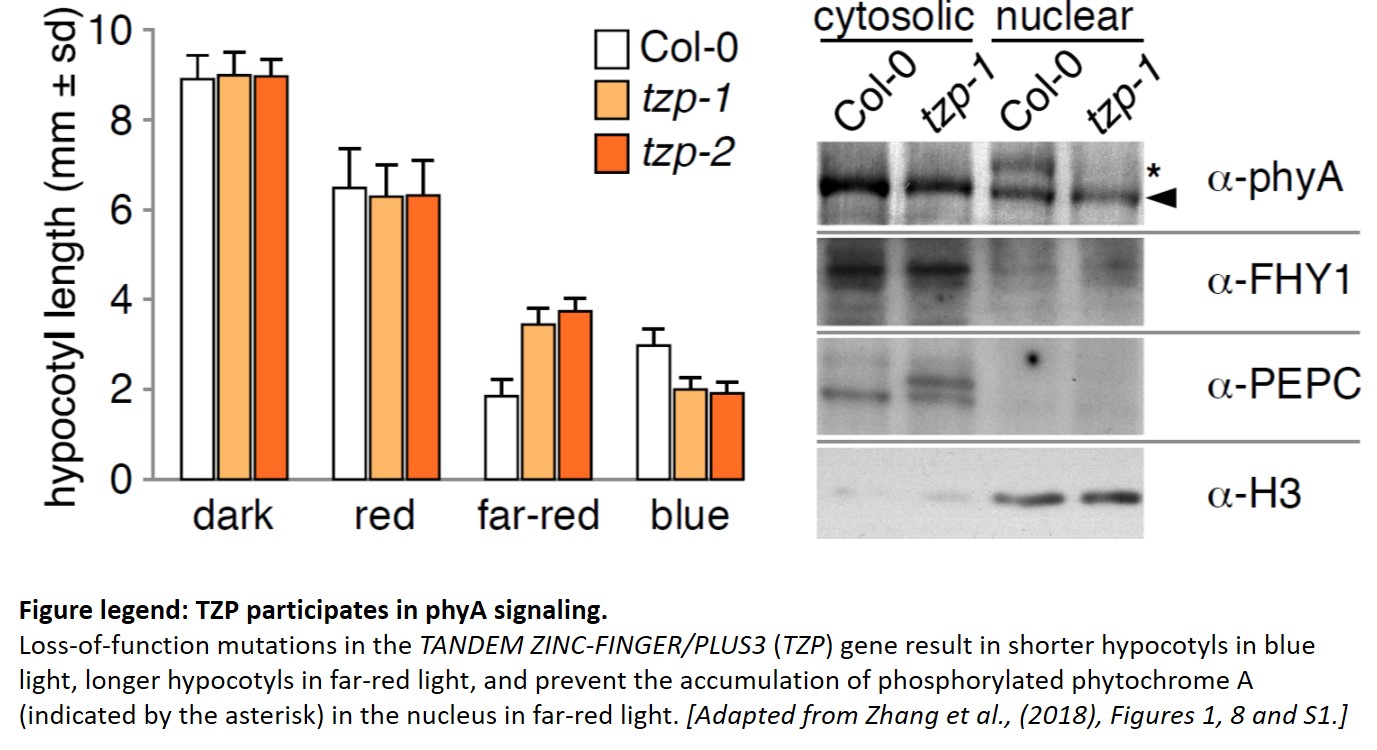 Since TZP was identified through natural variation, much of TZP’s function had been inferred from overexpression lines. The work from Zhang and colleagues provides the long-awaited characterization of tzp mutants in the reference accession Col-0, isolated from a forward genetic screen. tzp mutants have longer hypocotyls and accumulate less anthocyanins than wild-type when grown in far-red light, anchoring TZP as a positive regulator of phyA signaling (see Figure). But tzp seedlings also have shorter hypocotyls in blue light, as with the initial natural allele (Loudet et al., 2008), indicating an additional negative role in blue light signaling.
Since TZP was identified through natural variation, much of TZP’s function had been inferred from overexpression lines. The work from Zhang and colleagues provides the long-awaited characterization of tzp mutants in the reference accession Col-0, isolated from a forward genetic screen. tzp mutants have longer hypocotyls and accumulate less anthocyanins than wild-type when grown in far-red light, anchoring TZP as a positive regulator of phyA signaling (see Figure). But tzp seedlings also have shorter hypocotyls in blue light, as with the initial natural allele (Loudet et al., 2008), indicating an additional negative role in blue light signaling.
The role of TZP in phyA signaling comes into focus from biochemical experiments. First, TZP protein is phosphorylated in the light, and its modification pattern is distinct for red, far-red and blue light. Next, TZP interacts with phyA (and phyB) in vivo, with the phyA signaling component FAR-RED ELONGATED HYPOCOTYL-1 (FHY1), and controls the degradation of phyA and FHY1. Last but not least, phyA is phosphorylated in response to far-red light in the nucleus, but not in tzp mutants, where phyA exists only in its non-phosphorylated (and presumably less active) state. Phosphorylated phyA may be the preferred substrate for the E3 ubiquitin ligase CONSTITUTIVE PHOTOMORPHOGENIC-1 (COP1), explaining why more phyA accumulates in the tzp background in far-red light.
While it is now clear that TZP plays a role in phyA signaling, a few questions remain. Which kinase phosphorylates phyA? How can TZP be part of phyA and phyB signaling? It may all be a question of (developmental) timing: the TZP-phyA module will mediate responses to far-red light in seedlings growing under a canopy; later, after phyA levels have been depleted in the light, the TZP-phyB module takes over to control flowering time (Kaiserli et al., 2015). This would explain why tzp seedlings do not have a long hypocotyl in red light: it’s just not TZP’s time to shine in red light.
REFERENCES:
Kaiserli E, Páldi K, O’Donnell L, Batalov O, Pedmale UV, Nusinow DA, Kay SA, Chory J. Integration of Light and Photoperiodic Signaling in Transcriptional Nuclear Foci. Dev Cell 35: 311- 321.
Loudet O, Michael TP, Burger BT, Le Metté C, Mockler TC, Weigel D, Chory J (2008). A zinc knuckle protein that negatively controls morning-specific growth in Arabidopsis thaliana. Proc Natl Acad Sci. 105: 17193-17198.
Zhang S, Li C, Zhou Y, Wang X, Li H, Feng Z, Chen H, Qin G, Jin D, Terzaghi W, Gu H, Qu L-J, Kang D, Deng XW, Li J (2018). TANDEM ZINC-FINGER/PLUS3 is a key component of phytochrome A signaling. Published March 2018. DOI: https://doi.org/10.1105/tpc.17.00677.