Active Support: GHR1 is a Pseudokinase that acts as a Scaffolding Component
Plants balance CO2 uptake with water loss via a complex network of signals regulating stomatal aperture size. Stomata close in response to a number of stimuli, including drought, low light intensity, low air humidity, elevated intercellular CO2 concentration, pathogens, and certain air-borne chemicals or pollutants such as ozone. After plants perceive these triggers, multiple signaling cascades involving several kinases and channels ultimately lead to stomatal closure. The plasma membrane receptor kinase GUARD CELL HYDROGEN PEROXIDE RESISTANT1 (GHR1) was previously characterized to mediate abscisic acid (ABA) and hydrogen peroxide-regulated stomatal movements, and there is evidence that it functions by phosphorylating SLOW ANION CHANNEL1 (SLAC1) (Hua et al., 2012). Sierla et al. (2018) now show that GHR1 is in fact a pseudokinase and present an in-depth view of its role in stomatal closure.
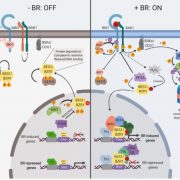
The authors found that the loss of GHR1 impairs stomatal closure induced by different stimuli; Arabidopsis thaliana ghr1 mutants have impaired responses to apoplastic reactive oxygen species induced by ozone, elevated CO2 and light-dark transitions and ABA. Using two independent forward-genetic ozone sensitivity screens, the authors identified new ghr1 alleles with mutations in various protein domains. GHR1 contains three predicted functional domains: the leucine-rich repeat, transmembrane, and kinase domains (see Figure).
The GHR1W799* mutation in the ghr1-17 allele introduces a stop codon leading to a truncated protein lacking most of the kinase domain, thereby representing a kinase-domain deficient ghr1 mutant (see Figure). This mutant had stomatal dysfunctions in response to a range of stimuli, but most phenotypes were not as severe as those of the ghr1-3 T-DNA mutant. The kinase domain of GHR1 fused to green fluorescent protein could not complement the ghr1-3 mutant, indicating that this domain alone is not sufficient for GHR1 function in planta.
Further exploration of the kinase domain revealed that GHR1 lacks two critical residues required for kinase function and demonstrated that GHR1 does not show in vitro kinase activity. The authors demonstrated that the intracellular domain of GHR1 can bind ATP in vitro and generated the mutant version GHR1K798W, which cannot bind ATP. GHR1K798W complemented the stomatal phenotypes of ghr1-3 in planta, indicating that ATP-binding and thus kinase activity are not required for GHR1 function in Arabidopsis.
The authors used heterologous expression in Xenopus oocytes to decipher GHR1-mediated SLAC1 activation mechanisms. Mutant versions of GHR1 that cannot bind ATP could still elicit anion currents when co-expressed with SLAC1. Mutations in the ghr1-7 and ghr1-8 alleles, located in the leucine-rich repeats of the GHR1 ectodomain (see Figure), were used to investigate whether these structural elements are required for SLAC1 activation. GHR1G108D and GHR1D293N could not interact with SLAC1, nor induce currents, in oocytes, indicating that these residues are required for the interaction, and activation, of SLAC1.

If GHR1 is a pseudokinase due to the absence of key residues essential for catalytic kinase activity, how does it influence SLAC1 activity? The authors hypothesize that GHR1 functions as a scaffold for additional regulators that convey the message to SLAC1. The authors identified CALCIUM-DEPENDENT PROTEIN KINASE3 (CPK3), previously shown to phosphorylate SLAC1 (Scherzer et al., 2012), as a potential GHR1 interactor.
While some components in stomatal signaling are stimulus-specific, GHR1 appears to play a central role due to its involvement in multiple stomatal responses. Sierla et al. provide evidence that GHR1 function is not due to inherent kinase activity. Rather, the combinatorial function involving its other protein domains and its interaction with CPK3 indicates that GHR1 likely plays an important scaffolding role in stomatal closure.
REFERENCES
Hua D, Wang C, He J, Liao H, Duan Y, Zhu Z, Guo Y, Chen Z and Gong Z (2012). A plasma membrane receptor kinase, GHR1, mediates abscisic acid- and hydrogen peroxide-regulated stomatal movement in Arabidopsis. Plant Cell 24: 2546-2561.
Scherzer S, Maierhofer T, Al-Rasheid KA, Geiger D and Hedrich R (2012). Multiple calcium-dependent kinases modulate ABA-activated guard cell anion channels. Mol Plant 5: 1409-1412.
Sierla M, et al. (2018). The receptor-like pseudokinase GHR1 is required for stomatal closure. Plant Cell 10.1105/tpc.18.00441.